Abstract
Purpose
Low-dose rivaroxaban is often given to patients with atrial fibrillation (AF) around the world, but the rationale for its use remains unclear. We aimed to compare the efficacy and safety of standard- or low-dose rivaroxaban in patients with AF through systematic review of literature with meta-analysis.
Methods
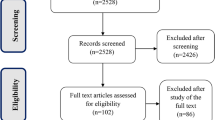
We searched PubMed, Web of Science, EMBASE, Clinical Trials.gov, the Cochrane Library, and Bayer trial website from inception of each database until June 2020. Randomized controlled trials (RCTs) and cohort studies were included in the meta-analysis. A random-effects model was employed to calculate the pooled effect estimates.
Results
Two RCTs and 17 cohort studies were included in the qualitative analysis. Indirect comparison of RCTs showed no significant difference between the two rivaroxaban dosages in risk of efficacy or safety outcomes (p > 0.05). Indirect comparison of cohort studies showed a lower risk of MACE among Caucasians in standard-dose group (HR 0.779; 95% CI 0.687–0.884; p < 0.001). Bleeding outcomes did not differ significantly between the two dosage regimens in Asian or Caucasian populations, except that the standard dose was associated with higher risk of major bleeding among elderly Caucasian patients (HR 1.329; 95% CI 1.141–1.547; p < 0.001). The quality of evidence was rated ranging from very low to low for all the efficacy and safety outcomes.
Conclusion
In Caucasians with AF, standard-dose rivaroxaban may prevent MACE significantly better than low-dose treatment. Further studies in Asians are needed to verify the advantages of the standard dose.



Similar content being viewed by others
References
Virani SS, Alonso A, Benjamin EJ et al (2020) Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 141(9):e139–e596. https://doi.org/10.1161/CIR.0000000000000757
Tse HF, Wang YJ, Ahmed Ai-Abdullah M et al (2013) Stroke prevention in atrial fibrillation–an Asian stroke perspective. Heart Rhythm 10(7):1082–1088. https://doi.org/10.1016/j.hrthm.2013.03.017
January CT, Wann LS, Calkins H et al (2019) 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 74(1):104–132
Patel MR, Mahaffey KW, Garg J,: ROCKET AF Investigators, et al (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365(10):883–891. https://doi.org/10.1056/NEJMoa1009638
Alamneh EA, Chalmers L, Bereznicki LR (2016) Suboptimal use of oral anticoagulants in atrial fibrillation: has the introduction of direct oral anticoagulants improved prescribing practices? Am J Cardiovasc Drugs 16:183–200
Nguyen E, White CM, Patel MR et al (2016) Doses of apixaban and rivaroxaban prescribed in real-world United States cardiology practices compared to registration trials. Curr Med Res Opin 32(7):1277–1279
Ogawa S, Ikeda T, Kitazono T et al (2014) Present profiles of novel anticoagulant use in Japanese patients with atrial fibrillation: insights from the Rivaroxaban Postmarketing Surveillance Registry. J Stroke Cerebrovasc Dis 23(10):2520–2526
Hori M, Matsumoto M, Tanahashi N et al (2012) Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation – the J-ROCKET AF study. Circ J 76:2104–2111
Lee HF, Chan YH, Tu HT et al (2018) The effectiveness and safety of low-dose rivaroxaban in Asians with non-valvular atrial fibrillation. Int J Cardiol 261:78–83
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 339:b2535. https://doi.org/10.1136/bmj.b2535
Bayer (2020) The Bayer Clinical Trials Registry and Results Database website, (n.d.). https://clinicaltrials.bayer.com/. Accessed 19 June 2020
Higgins JP, Altman DG, Gøtzsche PC et al (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343: d5928
Oremus M, Oremus C, Hall GB et al (2012) Inter-rater and test-retest reliability of quality assessments by novice student raters using the Jadad and Newcastle-Ottawa Scales. BMJ Open 2: e001368
Sanderson S, Tatt ID, Higgins JP (2007) Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol 36:666–676
Bucher HC, Guyatt GH, Griffith LE, Walter SD (1997) The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 50(6):683–691
Song F, Altman DG, Glenny AM, Deeks JJ (2003) Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ 326(7387):472
Guyatt G, Oxman AD, Akl EA et al (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64(4):383–394.
Alcusky M, Tjia J, McManus DD et al (2020) Comparative Safety and Effectiveness of Direct-Acting Oral Anticoagulants Versus Warfarin: a National Cohort Study of Nursing Home Residents. J Gen Intern Med 35(8):2329–2337
Amin A, Garcia Reeves AB, Li X et al (2019) Effectiveness and safety of oral anticoagulants in older adults with non-valvular atrial fibrillation and heart failure. PLoS One 14(3): e0213614.
Blin P, Fauchier L, Dureau-Pournin C et al (2019) Effectiveness and Safety of Rivaroxaban 15 or 20 mg Versus Vitamin K Antagonists in Nonvalvular Atrial Fibrillation. Stroke 50(9):2469–2476
Bonnemeier H, Huelsebeck M, Kloss S (2019) Comparative effectiveness of rivaroxaban versus a vitamin K antagonist in patients with renal impairment treated for non-valvular atrial fibrillation in Germany - A retrospective cohort study. Int J Cardiol Heart Vasc 23: 100367
Coleman CI, Turpie AGG, Bunz TJ et al (2019) Effectiveness and safety of rivaroxaban vs. warfarin in non-valvular atrial fibrillation patients with a non-sex-related CHA2DS2-VASc score of 1. Eur Heart J Cardiovasc Pharmacother 5(2): 64–69
Chan YH, Lee HF, Wang CL et al (2019) Comparisons of Rivaroxaban Following Different Dosage Criteria (ROCKET AF or J-ROCKET AF Trials) in Asian Patients With Atrial Fibrillation. J Am Heart Assoc 8(21): e013053.
Cho MS, Yun JE, Park JJ et al (2018) Outcomes After Use of Standard- and Low-Dose Non-Vitamin K Oral Anticoagulants in Asian Patients With Atrial Fibrillation. Stroke. https://doi.org/10.1161/STROKEAHA.118.023093
Deitelzweig S, Keshishian A, Li X et al (2019) Comparisons between Oral Anticoagulants among Older Nonvalvular Atrial Fibrillation Patients. J Am Geriatr Soc 67(8):1662–1671. https://doi.org/10.1111/jgs.15956.Erratum.In:JAmGeriatrSoc.2020;68(8):E43-E49
Fauchier L, Blin P, Sacher F et al (2020) Reduced dose of rivaroxaban and dabigatran vs. vitamin K antagonists in very elderly patients with atrial fibrillation in a nationwide cohort study. Europace 22(2): 205–215
Gorst-Rasmussen A, Lip GY, Bjerregaard Larsen T (2016) Rivaroxaban versus warfarin and dabigatran in atrial fibrillation: comparative effectiveness and safety in Danish routine care. Pharmacoepidemiol Drug Saf 25(11):1236–1244
Huang HY, Lin SY, Cheng SH et al (2018) Effectiveness and Safety of Different Rivaroxaban Dosage Regimens in Patients with Non-Valvular Atrial Fibrillation: A Nationwide. Population-Based Cohort Study Sci Rep 8(1):3451
Kohsaka S, Katada J, Saito K, et al (2020) Safety and effectiveness of non-vitamin K oral anticoagulants versus warfarin in real-world patients with non-valvular atrial fibrillation: a retrospective analysis of contemporary Japanese administrative claims data. Open Heart 7(1): e001232
Lip GYH, Keshishian AV, Kang AL et al (2020) Effectiveness and Safety of Oral Anticoagulants in Patients With Nonvalvular Atrial Fibrillation and Diabetes Mellitus. Mayo Clin Proc 95(5):929–943
Lip GYH, Skjøth F, Nielsen PB et al (2017) Effectiveness and Safety of Standard-Dose Nonvitamin K Antagonist Oral Anticoagulants and Warfarin Among Patients With Atrial Fibrillation With a Single Stroke Risk Factor: A Nationwide Cohort Study. JAMA Cardiol 2(8):872–881
Nielsen PB, Skjøth F, Søgaard M et al (2017) Effectiveness and safety of reduced dose non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ 356: j510
Pratt NL, Ramsay E, Kalisch Ellett LM et al (2019) Comparative effectiveness and safety of low-strength and high-strength direct oral anticoagulants compared with warfarin: a sequential cohort study. BMJ Open 9(5): e026486
Lee SR, Choi EK, Han KD et al (2019) Optimal Rivaroxaban Dose in Asian Patients With Atrial Fibrillation and Normal or Mildly Impaired Renal Function. Stroke 50(5):1140–1148
Camm AJ, Cools F, Virdone S, GARFIELD-AF Investigators et al (2020) Mortality in Patients With Atrial Fibrillation Receiving Nonrecommended Doses of Direct Oral Anticoagulants. J Am Coll Cardiol 76(12):1425–1436
Janssen Pharmaceuticals Inc (2021) XARELTO® (rivaroxaban) tablets, for oral use [package insert]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/022406s036,202439s036lbl.pdf. Accessed 21 February 2021
Lip GY, Frison L, Halperin JL, Lane DA (2011) Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: The HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly) score. J Am Coll Cardiol 57(2):173–180. https://doi.org/10.1016/j.jacc.2010.09.024
Ugowe FE, Jackson LR 2nd, Thomas KL (2018) Racial and ethnic differences in the prevalence, management, and outcomes in patients with atrial fibrillation: A systematic review. Hear Rhythm 15(9):1337–1345. https://doi.org/10.1016/j.hrthm.2018.05.019
Funding
This study was supported by grants from the National Key R&D Program of China (No. 2016YFC0904900), National Science and Technology Major Projects for “Major New Drugs Innovation and Development” of China (No. 2018ZX09201014, No. 2017ZX09101001 and No. 2017ZX09304028), National Natural Science Foundation of China (No. 81872940, 81973395 and 82073935) and Natural Science Foundation of Beijing Municipality (No. 7171012).
Author information
Authors and Affiliations
Contributions
Designed research: Guangyan Mu, Hanxu Zhang, Qian Xiang, and Yimin Cui.
Performed research: Guangyan Mu, Hanxu Zhang, Zhiyan Liu, Qiufen Xie, and Zining Wang.
Analyzed data: Guangyan Mu, Shuang Zhou, and Zhe Wang.
Wrote the manuscript: Guangyan Mu, and Hanxu Zhang.
Critically reviewed the manuscript: Kun Hu, Jingyi Hou, Nan Zhao, Qian Xiang, and Yimin Cui.
All authors approved the submitted version of the manuscript.
Corresponding authors
Ethics declarations
Conflicts of interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mu, G., Zhang, H., Liu, Z. et al. Standard- vs. low-dose rivaroxaban in patients with atrial fibrillation: a systematic review and meta-analysis. Eur J Clin Pharmacol 78, 181–190 (2022). https://doi.org/10.1007/s00228-021-03226-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03226-6